Arcellx´ CART-ddBCMA: a new player in the BCMA space
Latest results showed a 100% response rate in Ph1 trial and put Arcellx´s platform in direct competition with Legend Biotech Carvykti.
(Link for a shared folder with in-depth summaries at the end)
Highlights:
Arcellx provided a clinical update on its BCMA CAR-T, CART-ddBCMA.
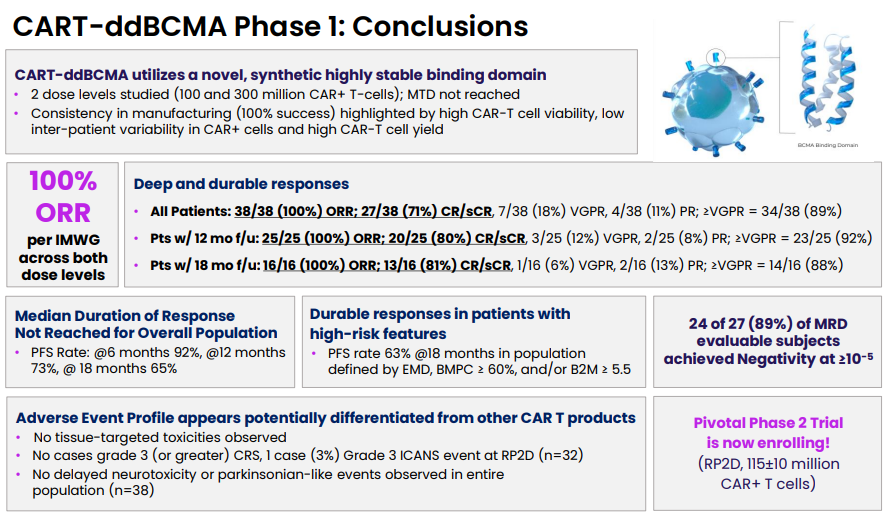
N=38 patients; 100% ORR, with 71% CR/sCR, 18% VGPR and 11% PR. RP2D was established and pivotal phase 2 has started.
Kite and Arcellx announced a co-development partnership for the CART-ddBCMA program.
On Friday, December 9th, Arcellx reported updated results on its BCMA-directed CAR-T program CART-ddBCMA, ahead of the ASH22 presentation, followed by a more in-depth presentation on Sunday. It also announced a strategic partnership with Kite Pharma, which will be in charge of manufacturing and commercialization (U.S.: co-commercialized by Arcellx/Kite; Ex-U.S.: commercialized by Kite only). As usual, comprehensive coverage of the deal was provided by Endpoint News, and more details about it can be found in the official press release (see summary slide below).
Most CARs use a scFv as an extracellular domain to engage the target antigen. However, as already discussed in this blog, some scFvs tend to aggregate and induce tonic signaling independent of the target. This can induce premature T-cell exhaustion and decrease the overall efficiency of the therapy. In addition, these molecules can be immunogenic as most antibodies are of mouse origin. scFv humanization may decrease its immunogenicity, but it needs to be evaluated on a case-by-case basis.
Arcellx´s approach to target BCMA involves the use of a D domain as an extracellular region instead of an scFv. In theory, this domain would have benefits such as decreased immunogenicity and decreased tonic signaling due to less aggregation. Also, it might help boost T cell transduction rates since it is smaller than a scFv (1/3 the size). In fact, the paper describing the preclinical development of CART-ddBCMA demonstrated better transduction efficiency and higher ddBCMA CAR expression when compared to the scFv-based Abecma construct (bb2121). However, when both CAR constructs were evaluated in in vivo experiments, they showed a similar antitumor response, making it unclear if it really had an edge against its competitors.
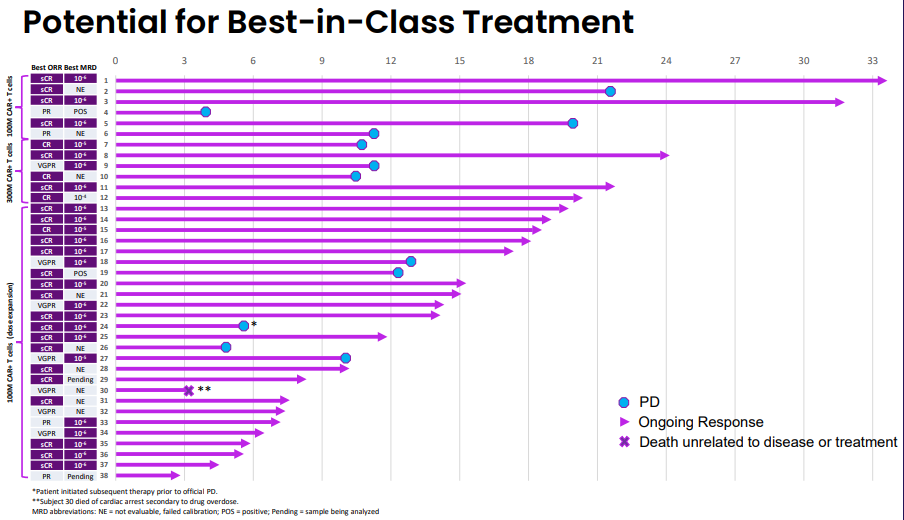
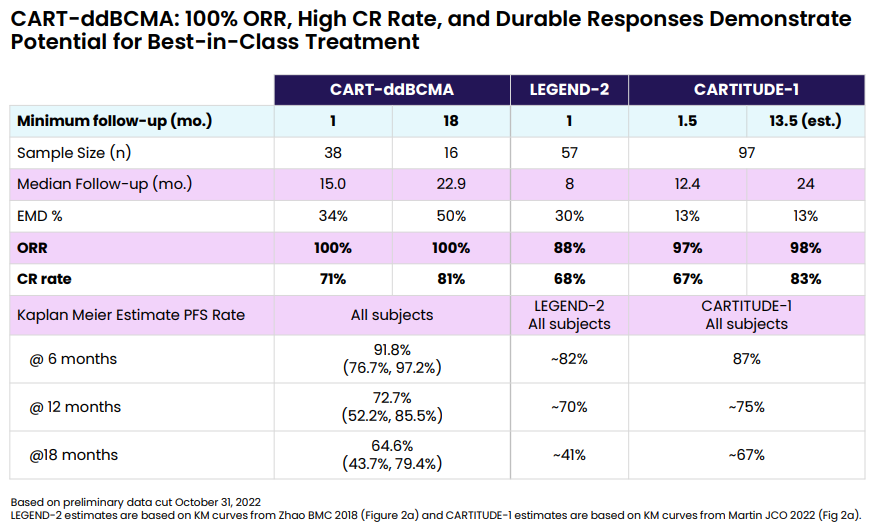
At ASH2022, clinical data supporting Arcellx's approach were presented, obtaining a remarkable 100% overall response rate (ORR) and 71% CR/sCR response (n=38) (Link to full presentation). The responses so far seem durable, with a PFS of 73% at 12 months. This was achieved in a heavily pretreated (median of 4 prior treatments), high-risk patient population, making the results even more encouraging. Somewhat surprisingly, no cases of grade 3 (or greater) CRS cases were observed, and only 1 patient experienced a grade 3 ICANS. Importantly, no parkinsonian-like event was observed.
Cross-trial comparisons are limited, especially in the case of multiple myeloma where so many patients exhibit a high-risk profile. However, the clinical data shows that CART-ddBCMA can be a potential best-in-class program, showing responses on the same level as Legend´s Carvykti.
So far, it is unclear why CART-ddBCMA has such high efficacy while keeping the CRS and ICANS numbers low. This was a point raised during the roundtable discussion, and Arcellx pointed out that this could be related to the lower tonic signaling of the D domain-based CAR. Arcellx, however, did not provide any data on it, nor on CAR-T expansion levels or anti-CAR antibodies, making it difficult to draw any firm conclusions.
It´s worth mentioning that similar strategies using alternative binders were tried before, but obtained limited success. Poseida Therapeutics developed an autologous anti-BCMA CAR-T program with an alternative, smaller binder. P-BCMA-101 used a Centyrin-based target recognition domain, also with the aim of reducing immunogenicity and decreasing tonic signaling. However, anti-CAR antibodies were still observed in the patients and, due to lower-than-expected efficacy, the program was terminated. Its successor, the allogeneic P-BCMA-ALLO1 program, uses a heavy chain (VH)-only binder, which is more structurally related to Legend´s Carvykti construct.
Kite has been pursuing a BCMA-directed CAR-T program for quite a while now. KITE-585, a 2nd Gen CAR-T using a fully-human scFv, CD28, and CD3zeta domains, was terminated in 2019 due to lack of efficacy after the Phase 1 trial. A detailed study was published in 2021, indicating that KITE-585 failed to expand in patients and that the observed partial responses were likely due to bridging therapy.
In 2020, Kite established a collaboration with Teneobio and received exclusive rights to a fully-human, heavy chain (VH)-only anti-BCMA binder (FHVH33) that is currently being evaluated by the NCI in a phase 1 trial (NCT03602612). Interestingly, the latest results of this trial were also reported at ASH22, showing a 92% ORR (N=25). However, 6/25 patients experienced grade 3 CRS (Lee criteria) and 8/25 patients had neurologic toxicity (6 patients with grade 2 and 2 patients with grade 3; CTCAE 5.0). In addition, anti-CAR humoral immune responses were observed in 4 of 12 patients, invalidating the hypothesis that a fully-human, smaller binder would decrease or eliminate potential immunogenicity.
Despite being unclear whether it is the main factor responsible for the program's success, Arcellx's results show that its bet on D-domain technology paid off. The results were promising enough to convince Kite to enter into a strategic partnership, and compared to FHVH33 and other BCMA CAR programs, it has a clear safety advantage. The deal also validates Arcellx´s platform and may also help to bring this therapy faster to patients given Kite´s manufacturing capabilities and experience.
Shared folder containing summaries for anti-BCMA CAR-T programs discussed in the post: https://drive.google.com/drive/folders/1vvn6MS5Lbng-cQh6KREOgjw5qO9EkOyq?usp=sharing
CART-ddBCMA (Arcellx)
KITE-585 (Kite)
FHVH33 (NCI)